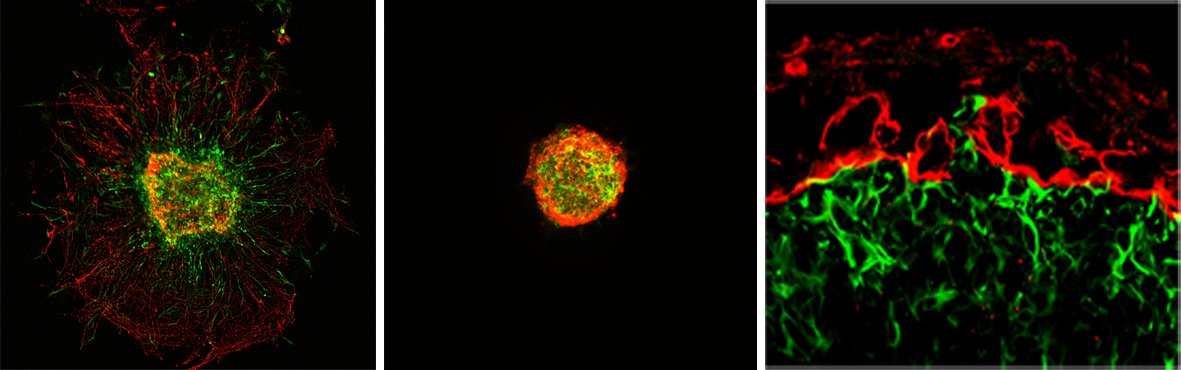
Central Nervous System (CNS) Myelination
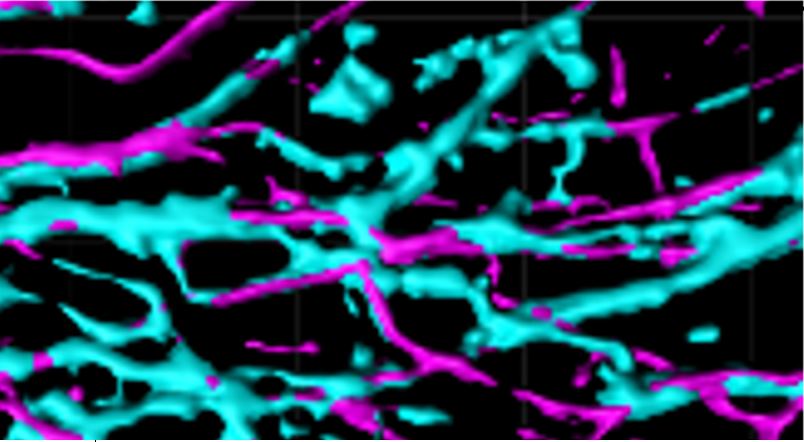
Myelin is a specialized multilamellar membrane structure that is formed by oligodendrocytes (OLs) of the CNS. OLs follow an orderly and distinct developmental pattern with stage-specific functions, including OL precursor cell (OPC) proliferation, pre-myelinating OL (pmOL) ensheathment of axons, and myelinating OL (mOL) iterative wrapping of axons. Cytoskeletal rearrangements play an important but different role at each stage. Actin filament (F-actin) formation is essential for axon ensheathment in pmOLs, whereas F-actin disassembly is required for mOL sto wrap axons. Ongoing research efforts aim to elucidate molecular mechanisms underlying pmOL biology, axon ensheathment, and their implications in myelin-related diseases, including developmental white matter injury and multiple sclerosis.
Neuro-immune interplays during interneuron development
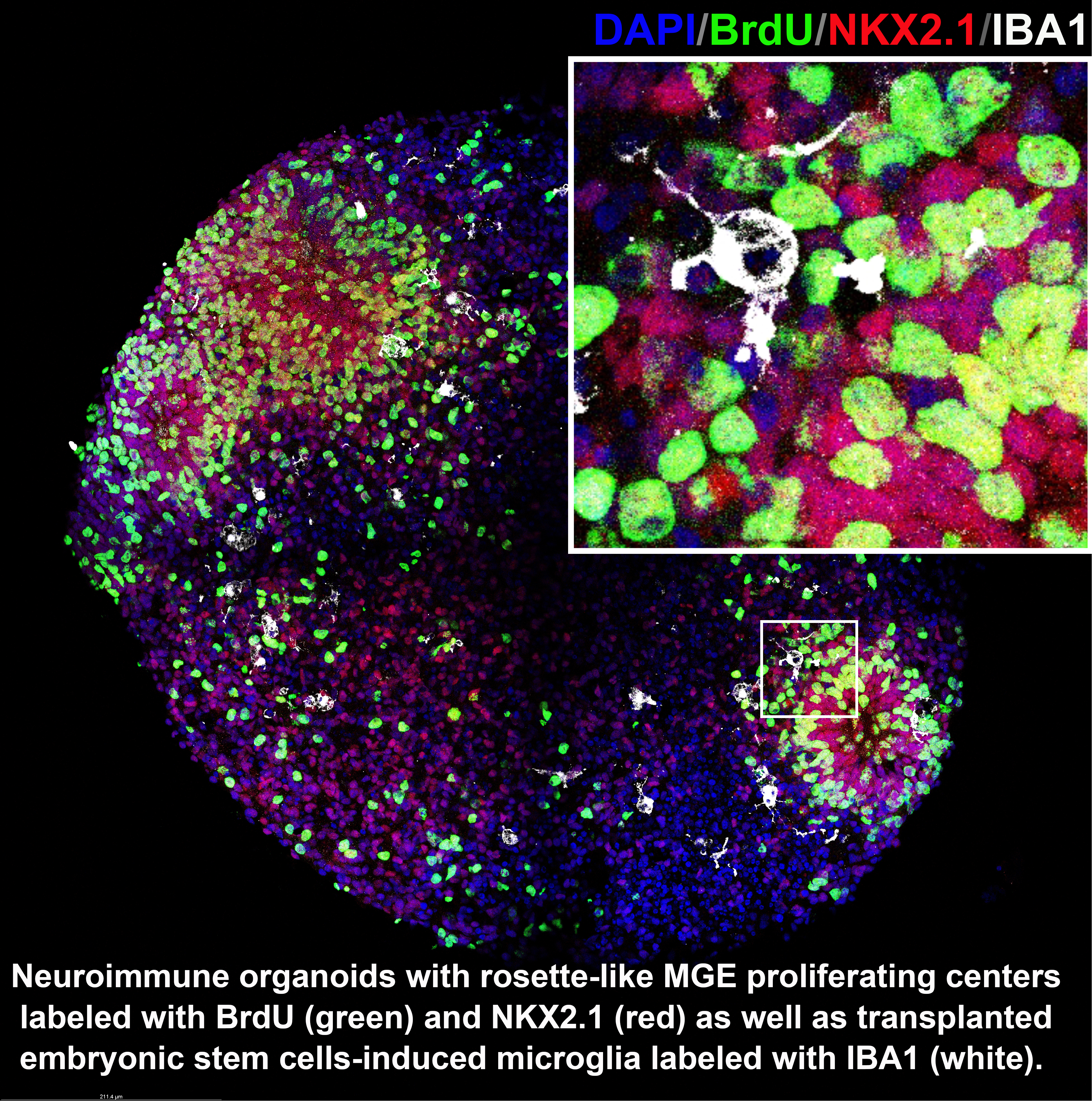
Cortical interneurons provide the main cortical inhibitory input and play a critical role in maintaining circuit rhythm and excitation-inhibition balance in the brain. Cortical interneuron deficits have been implicated in multiple neurological disorders, including autism spectrum disorders, schizophrenia, and Alzheimer's disease. To investigate how glia regulate interneuron development in humans, we generated a single-nucleus transcriptomics database from perinatal post-mortem human samples and performed cell-cell interaction analyses. We found that microglia played an essential role in the development of human interneurons. We are establishing neuro-immune organoids as in vitro models to study the interaction between microglia and interneurons.
The impact of maternal immune activation on brain development
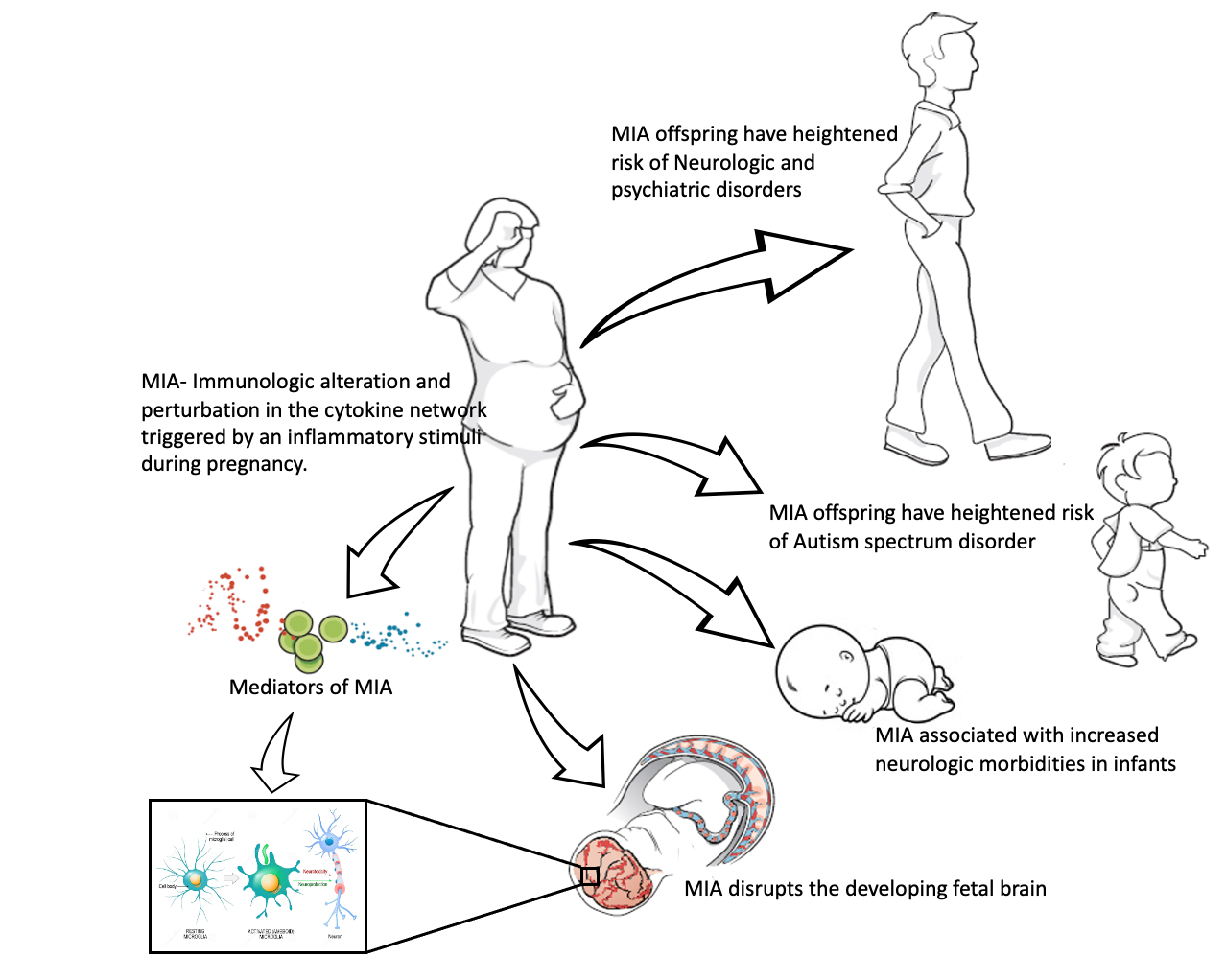
Maternal immune activation (MIA) can be defined as an immunological alteration triggered by an inflammatory stimulus during pregnancy. Epidemiological and animal model studies have found MIA to be associated with neuropsychiatric disorders in offspring like autism spectrum disorders, attention deficit hyperactivity disorder (ADHD), and schizophrenia. Additionally, results from our epidemiological study found MIA to be associated with neurological morbidities in offspring, such as periventricular leukomalacia (PVL), seizures, and abnormal neurological exams, as early as the neonatal period. These results suggest a disruption in the fetal neurodevelopmental processes secondary to MIA. However, the mechanisms through which MIA perpetuates its impact remain poorly understood. Utilizing translational research techniques, we plan to identify the specific mediators of MIA that create these neurodevelopmental morbidities in offspring. Ongoing research aims to uncover the cellular and molecular mechanisms underlying MIA-mediated neurodevelopmental disruption. Such an understanding will provide therapeutic and preventative opportunities to mitigate the most detrimental impacts of MIA on the developing fetal brain.
Molecular mechanisms underlying microglia-mediated synaptic pruning
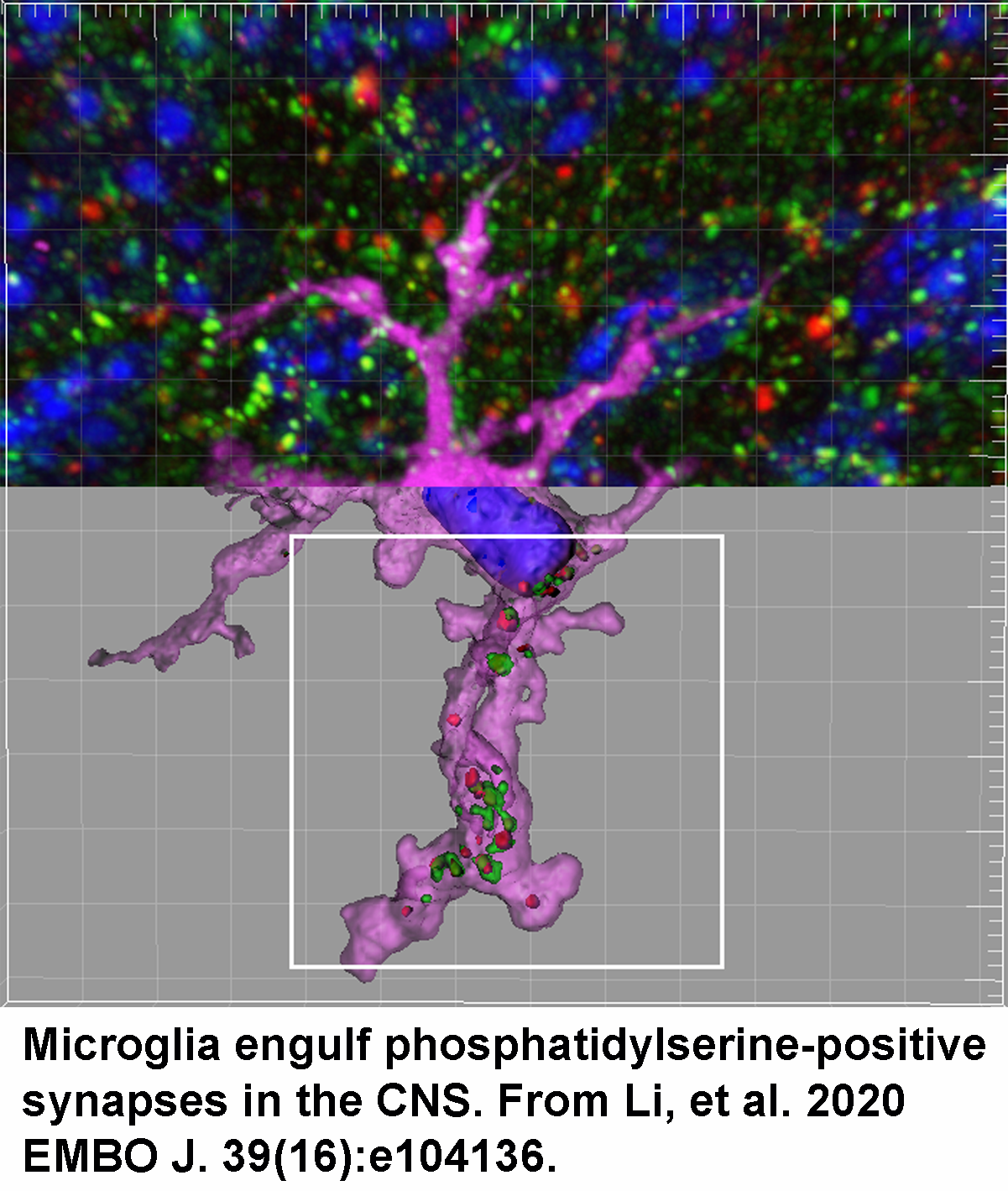
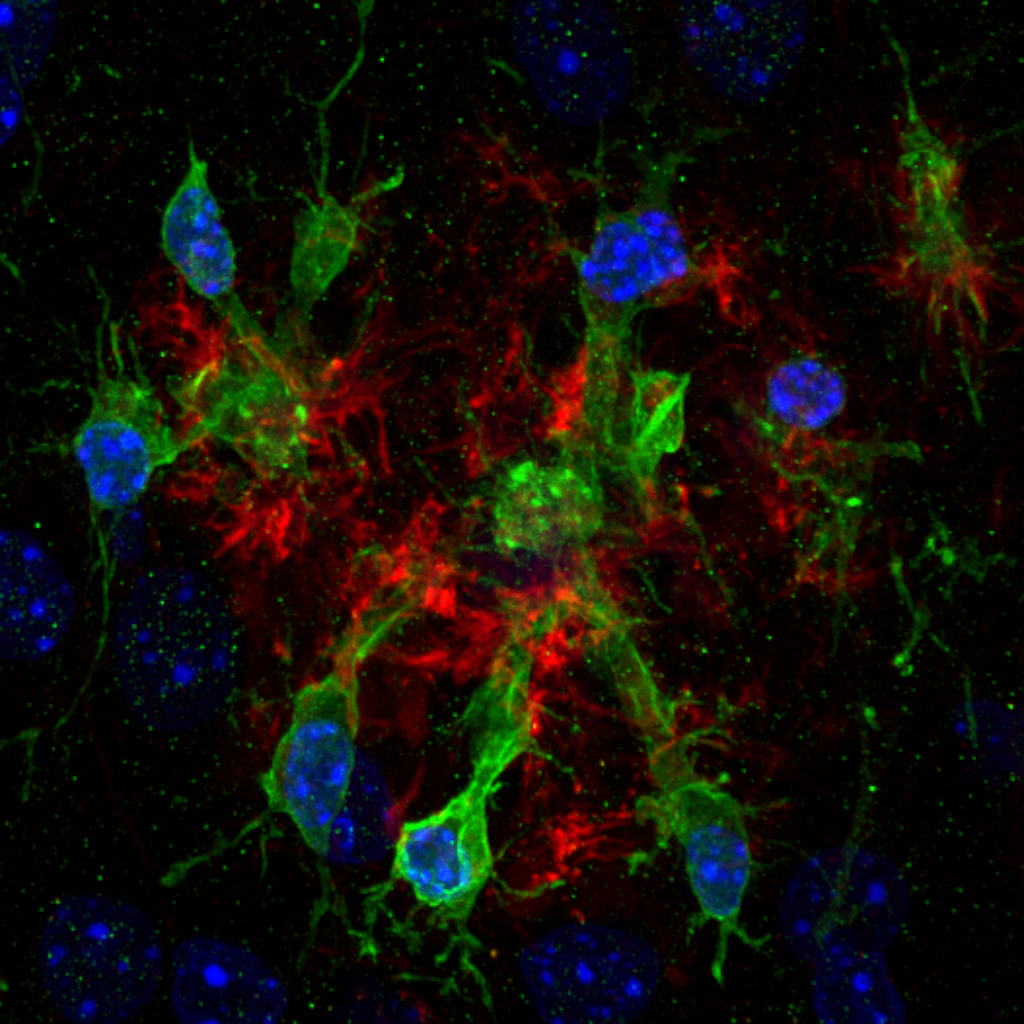
Microglia are the main tissue-resident macrophages of the CNS and regulate a variety of CNS processes, including neurodevelopment, the maintenance of neuronal networks, and injury repair. Their dysfunction has been linked to neurodevelopmental disorders like autism spectrum disorders and schizophrenia. Two major focuses of our research are to understand how microglia interact with neurons to regulate neuronal networks, and to further identify molecules that mediate synaptic pruning. One of our recent discoveries is that microglia prune synapses by recognizing phosphatidylserine (PS), which is externalized on synapse membranes via flippases. Our current research has two primary aims: (1) We hope to study the downstream signaling pathway of how microglia react when interacting with PS, and to further characterize the underlying synaptic mechanism that regulates PS externalization. (2) Most synaptic pruning-related work is focused on animal models due to the limited access to human tissues. To address this, the Piao Lab is establishing a human brain organoid model that will enable us to accurately study synaptic pruning.
Glial-glial and glial-neuronal interactions in Alzheimer's disease
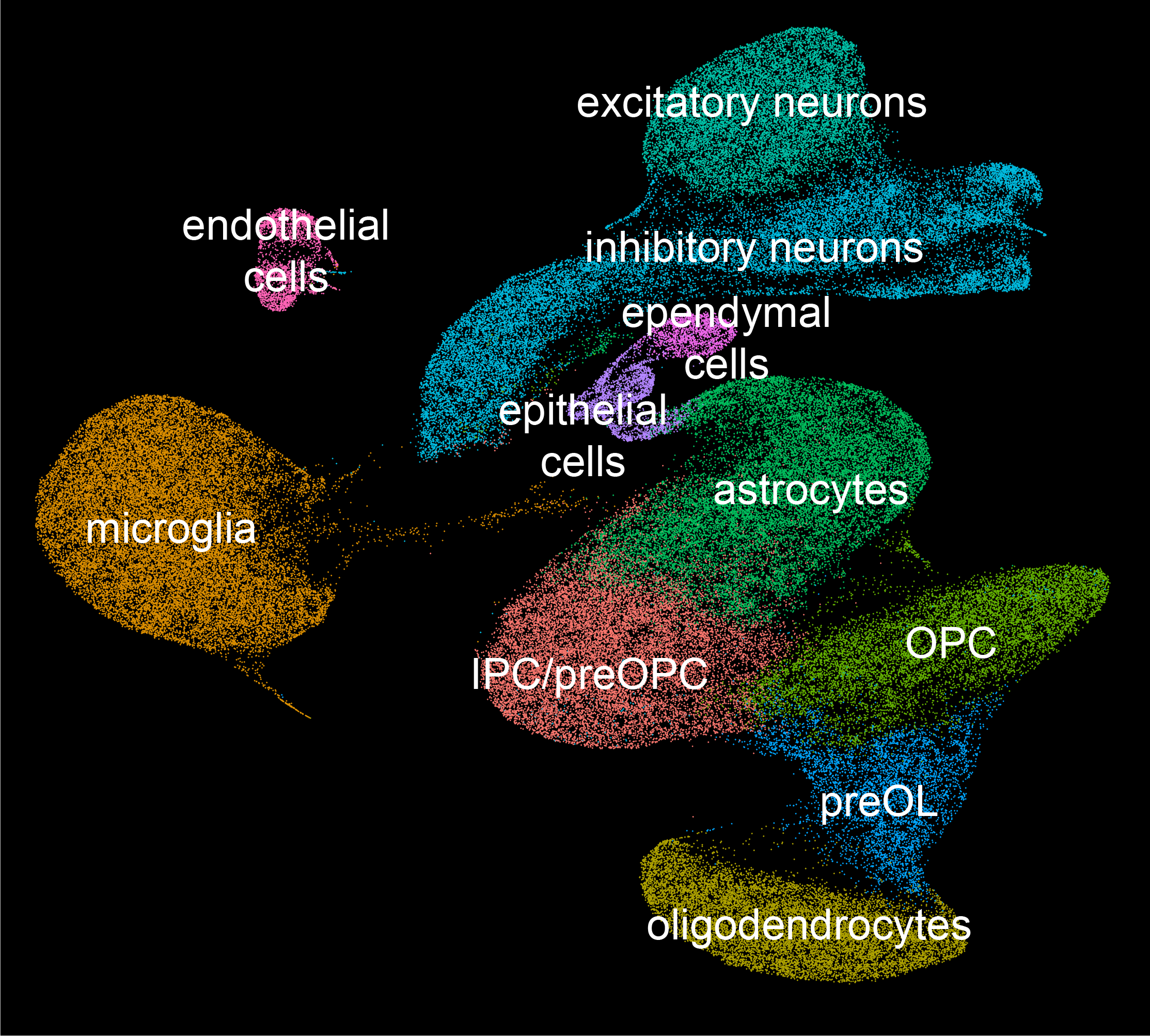
Glial cells play key functions during brain homeostasis and their dysfunction contributes to neurodegeneration such as Alzheimer's disease (AD). Our ongoing research investigates glial contributions to AD pathology. We generated single-nucleus RNA-sequencing (snRNA-seq) datasets in mouse transgenic models. Additionally, our lab is planning on generating snRNA-seq datasets for brain samples from post-mortem humans with AD. Previously published computational algorithms on inferring cell-cell interactions between different cell types will be used to identify and evaluate the glial interactions responsible for AD development. We also plan on utilizing spatial transcriptomic profiling methods to identify local cell-cell interactions.